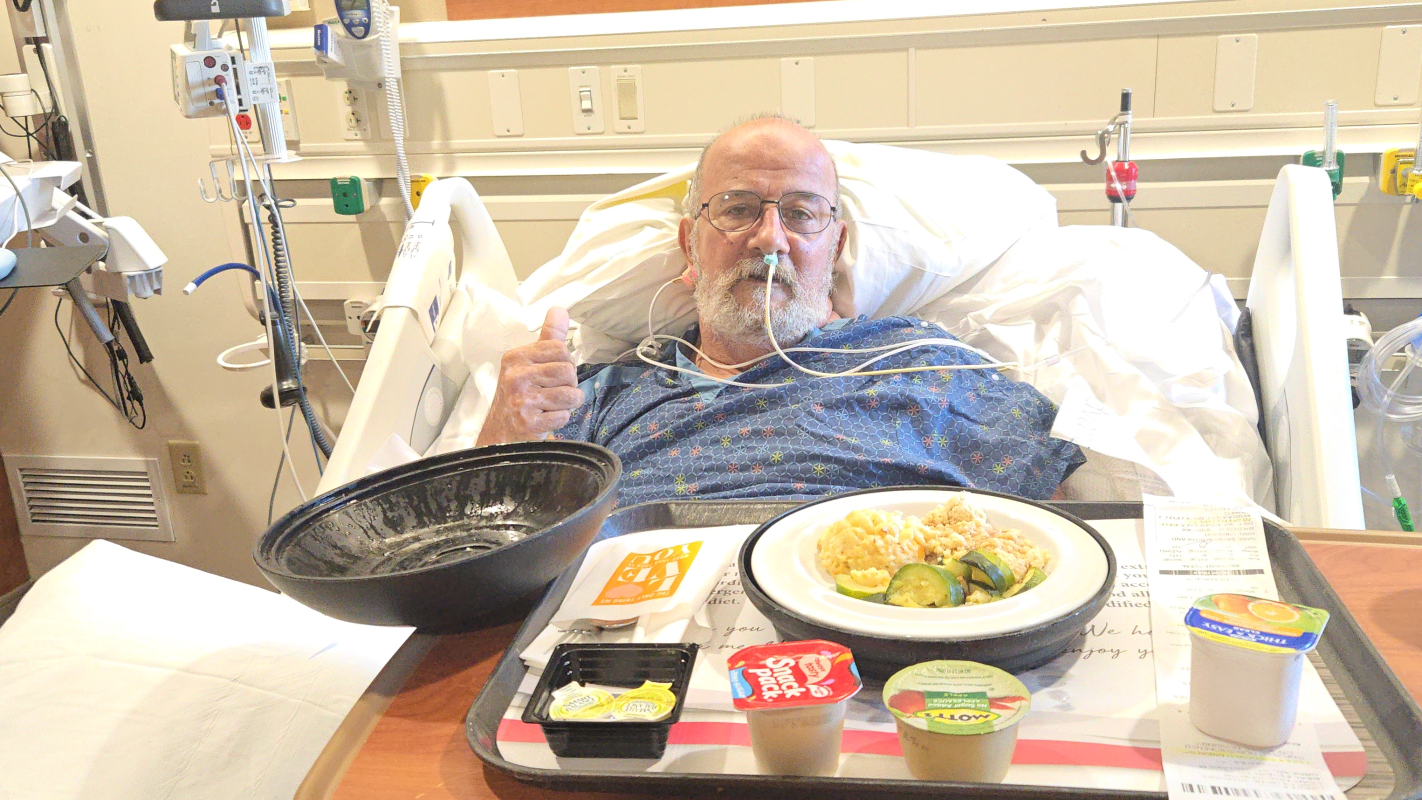
Our beloved Arne, a U.S. Navy Veteran, devoted husband to Joanne, and proud father of Bryan (23) and Zackary (13), is in the fight of his life.
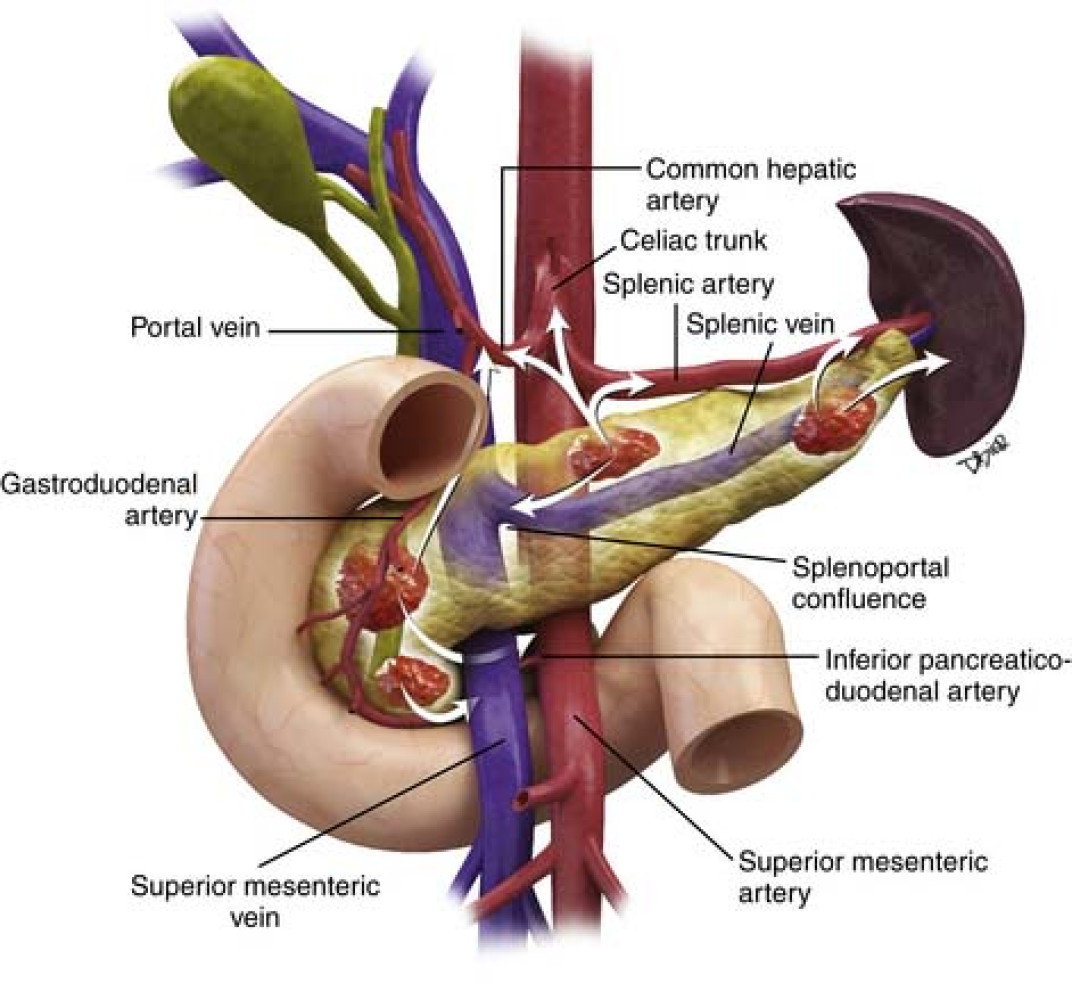
He has been diagnosed with Stage IV pancreatic cancer, which has spread to his liver and lymph nodes. His tumor is currently unresectable due to severe encasement around major arteries and veins, making surgery impossible at this stage.
Despite the grim statistics — with an average 5-year survival rate of less than 3% for Stage IV pancreatic cancer — we have found a glimmer of hope through a groundbreaking opportunity: the GANNON Clinical Trial in Barcelona, Spain.
This trial is testing histotripsy, a revolutionary, non-invasive therapy that uses focused ultrasound waves to destroy cancer cells — without radiation or chemotherapy. Histotripsy has already been FDA-approved for liver tumors, showing promising results in early studies, with some patients experiencing significant tumor reduction or elimination while preserving healthy tissue.
The GANNON trial is the first-ever clinical study applying histotripsy to pancreatic cancer, offering hope where conventional treatments have failed.
While the trial itself is free, the associated medical expenses are not. Arne will need to be hospitalized in Spain, and the estimated costs for hospitalization, ICU care, and other related medical needs is expected to be up to €50,000, not including airfare, lodging, or food.
Arne has spent his life serving others — first as a U.S. Navy Veteran, and now as a board member of Hosanna Ministry, helping to support religious sisters and nuns from persecuted countries. His faith, compassion, and strength have touched so many lives. Now, we are asking for help to give him the chance to continue his fight — and his mission.
We are humbly reaching out to our friends, family, and community to help make this life-saving journey possible. Every bit truly counts — whether through a donation, sharing this campaign, or lifting us up in prayer. Your support brings us one step closer to giving Arne more time with his family and the possibility of healing. Any unused funds will be donated to pancreatic cancer research.
We are deeply grateful for your kindness, generosity, and continued prayers. During this most difficult time, we have felt surrounded by incredible love and support — and for that, we are profoundly blessed.
Thank you for standing with Arne and our family.
🙏 From the bottom of our hearts,
Joanne, Bryan, Zackary & the entire Gorney family
For Wise, PayPal, Zelle, or Venmo transfers, please use [email protected].






Please donate to comment.
{{dame(anonymous, user, donor_name)}} | {{curr(amount,'USD')}}
{{created_at_rel}}{{note}}